Intro
I work with stem cells in the lab, so I want to share the information on that topic at that blog. There are lots of interesting articles and study materials about stem cells on the web that's why I'm trying to gather them all here.
Showing posts with label embryo. Show all posts
Showing posts with label embryo. Show all posts
2/15/2013
2/07/2013
3-D printers can produce gun parts, aircraft wings, food and a lot more, but this new 3-D printed product may be the craziest thing yet: human embryonic stem cells.
Rebecca Boyle - Popular Science
3-D printers can produce gun parts, aircraft wings, food and a lot more, but this new 3-D printed product may be the craziest thing yet: human embryonic stem cells. Using stem cells as the "ink" in a 3-D printer, researchers in Scotland hope to eventually build 3-D printed organs and tissues. A team at Heriot-Watt University used a specially designed valve-based technique to deposit whole, live cells onto a surface in a specific pattern.
This article originally appeared at Popular Science.
The cells were floating in a "bio-ink," to use the terminology of the researchers who developed this technique. They were able to squeeze out tiny droplets, containing five cells or fewer per droplet, in a variety of shapes and sizes. To produce clumps of cells, the team printed out cells first and then overlaid those with cell-free bio-ink, resulting in larger droplets or spheroids of cells. The cells would group together inside these spheroids. Spheroid size is key, because stem cells need certain conditions to work properly. This is why very precisely controlled 3-D printing could be so valuable for stem cell research.
After being squeezed out of a thin valve, the cells were still alive and viable, and able to transform into any other cell in the body, the researchers say. It's the first time anyone has printed human embyronic stem cells, said lead researcher Will Wenmiao Shu, a professor at Heriot-Watt. But ... why?
Eventually, they could be used to print out new tissues, or as filler inside existing organs, which would be regenerated. It could even serve to limit animal testing for new drug compounds, allowing them to be tested on actual human tissue, said Jason King, business development manager at Roslin Cellab, one of the research partners. "In the longer term, [it could] provide organs for transplant on demand, without the need for donation and without the problems of immune suppression and potential organ rejection," he said in a statement.
The team took stem cells from an embryonic kidney and from a well-studied embryonic cell line, and grew them in culture. They had to build a custom reservoir — let's call it an inkwell — to safely house the delicate cells, and then they added some large-diameter nozzles. A pressurized air supply pumps the cells from the inkwell into the valves, which contain pressurized nozzles on the end. The team could control the amount of cells dispensed by changing any of the factors, including the pneumatic pressure, nozzle diameter or length of time the nozzle stayed open.
At first the researchers printed droplets, but ultimately, they were so precise that they made cell spheroids in a variety of shapes and sizes, like the university logo above. One interesting wrinkle: The cells also formed spheroids in the inkwells. More work needs to be done to explain that.
The researchers also took several steps to make sure the cells survived the printing process. Examining the results of several experiments, they found 99 percent of the cells were still viable after running through the valve-based printer. "This confirms that this printing process did not appear to damage the cells or affect the viability of the vast majority of dispensed cells," they write in their paper, which is being published in the IOP regenerative medicine journal Biofabrication.
Stem cells are powerful because they can develop into any cell in the body. Embryonic stem cells, which are taken from human embryos in the earliest stages of development, can be developed into stem cell lines that can be grown indefinitely. This is kind of controversial, especially in the US. But medical researchers think they could be hugely promising for a whole host of human ailments — stem cells could differentiate into neurons, potentially replacing the ones lost in degenerative diseases like Alzheimer's; or they could differentiate into pancreatic cells, curing diabetes; and so on.
Using a 3-D printer to produce gun parts has been pretty controversial, especially during the ongoing post-Connecticut-shooting gun debate. But that may be nothing compared to this.
http://io9.com/5981832/a-3d-printer-that-generates-human-embryonic-stem-cells
3-D printers can produce gun parts, aircraft wings, food and a lot more, but this new 3-D printed product may be the craziest thing yet: human embryonic stem cells. Using stem cells as the "ink" in a 3-D printer, researchers in Scotland hope to eventually build 3-D printed organs and tissues. A team at Heriot-Watt University used a specially designed valve-based technique to deposit whole, live cells onto a surface in a specific pattern.
This article originally appeared at Popular Science.
The cells were floating in a "bio-ink," to use the terminology of the researchers who developed this technique. They were able to squeeze out tiny droplets, containing five cells or fewer per droplet, in a variety of shapes and sizes. To produce clumps of cells, the team printed out cells first and then overlaid those with cell-free bio-ink, resulting in larger droplets or spheroids of cells. The cells would group together inside these spheroids. Spheroid size is key, because stem cells need certain conditions to work properly. This is why very precisely controlled 3-D printing could be so valuable for stem cell research.
After being squeezed out of a thin valve, the cells were still alive and viable, and able to transform into any other cell in the body, the researchers say. It's the first time anyone has printed human embyronic stem cells, said lead researcher Will Wenmiao Shu, a professor at Heriot-Watt. But ... why?
Eventually, they could be used to print out new tissues, or as filler inside existing organs, which would be regenerated. It could even serve to limit animal testing for new drug compounds, allowing them to be tested on actual human tissue, said Jason King, business development manager at Roslin Cellab, one of the research partners. "In the longer term, [it could] provide organs for transplant on demand, without the need for donation and without the problems of immune suppression and potential organ rejection," he said in a statement.
The team took stem cells from an embryonic kidney and from a well-studied embryonic cell line, and grew them in culture. They had to build a custom reservoir — let's call it an inkwell — to safely house the delicate cells, and then they added some large-diameter nozzles. A pressurized air supply pumps the cells from the inkwell into the valves, which contain pressurized nozzles on the end. The team could control the amount of cells dispensed by changing any of the factors, including the pneumatic pressure, nozzle diameter or length of time the nozzle stayed open.
At first the researchers printed droplets, but ultimately, they were so precise that they made cell spheroids in a variety of shapes and sizes, like the university logo above. One interesting wrinkle: The cells also formed spheroids in the inkwells. More work needs to be done to explain that.
The researchers also took several steps to make sure the cells survived the printing process. Examining the results of several experiments, they found 99 percent of the cells were still viable after running through the valve-based printer. "This confirms that this printing process did not appear to damage the cells or affect the viability of the vast majority of dispensed cells," they write in their paper, which is being published in the IOP regenerative medicine journal Biofabrication.
Stem cells are powerful because they can develop into any cell in the body. Embryonic stem cells, which are taken from human embryos in the earliest stages of development, can be developed into stem cell lines that can be grown indefinitely. This is kind of controversial, especially in the US. But medical researchers think they could be hugely promising for a whole host of human ailments — stem cells could differentiate into neurons, potentially replacing the ones lost in degenerative diseases like Alzheimer's; or they could differentiate into pancreatic cells, curing diabetes; and so on.
Using a 3-D printer to produce gun parts has been pretty controversial, especially during the ongoing post-Connecticut-shooting gun debate. But that may be nothing compared to this.
http://io9.com/5981832/a-3d-printer-that-generates-human-embryonic-stem-cells
8/27/2012
.
Here is the express Stem Cell news on the other place - my paper.li. Very interesting place to put your information. I like it!:
5/26/2012
Stem Cell Comics
Open publication - Free publishing
Beautiful educational comics about stem cells! ‘Hope Beyond Hype’ is a story about stem cell therapies from science discovery to working therapy.
4/16/2012
Cool Article About the History of Stem Cell Research!
That's wonderful! I thought about it lots of times!
Who really discovered stem cells?
Is it even possible that one scientific team all by themselves discovered something so ubiquitous as stem cells?
In theory “yes”, but after much historical research including this great historical article in Cell Stem Cell, I would argue that no one group really discovered stem cells.
Instead I believe the “discovery” of stem cells was an ongoing team effort over a period of many decades and there is much credit to go around.
Who gets the credit now according to most people now for “discovering” stem cells?
Canada rightly takes pride in the work of their scientists Drs. James Till and Ernset McCulloch, who did pioneering studies in hematopoietic stem cell research.
In Canada, Till and McCulloch are unambiguously called the world’s discoverers of stem cells. Period. No ambiguity.
But is that correct?
Nope.
1/22/2012
Sperm from the Artificial Testicle!
Stem cell researchers are going to develop in vitro an important part of male's organism - testicle with sperm! More about the event was written by: Rachael Rettner, MyHealthNewsDaily Staff Writer
Published: 01/18/2012 05:20 PM EST on MyHealthNewsDaily
Researchers in California hope to become the first in the world to build an artificial testicle that produces human sperm. Such a device could allow infertile men to conceive children.
While recent studies have shown it's possible to treat infertile male mice by producing sperm using stem cells from the mouse, the same has not been done for humans, said researcher Dr. Paul Turek, director of the Turek Clinic, a men's health medical practice in San Francisco.
Using a newly received government grant, Turek and his fellow researchers hope to develop a human "sperm-making biological machine," he said.
Unlike a non-sperm-producing prosthesis — a saline-filled implant for men missing a testicle — the device will not be designed to resemble a testicle. Instead it will most closely resemble a cylindrical bag a few inches long, Turek said, creating a final product that looks something like a transparent, over-sized Tootsie Roll.
Published: 01/18/2012 05:20 PM EST on MyHealthNewsDaily
Researchers in California hope to become the first in the world to build an artificial testicle that produces human sperm. Such a device could allow infertile men to conceive children.
While recent studies have shown it's possible to treat infertile male mice by producing sperm using stem cells from the mouse, the same has not been done for humans, said researcher Dr. Paul Turek, director of the Turek Clinic, a men's health medical practice in San Francisco.
Using a newly received government grant, Turek and his fellow researchers hope to develop a human "sperm-making biological machine," he said.
Unlike a non-sperm-producing prosthesis — a saline-filled implant for men missing a testicle — the device will not be designed to resemble a testicle. Instead it will most closely resemble a cylindrical bag a few inches long, Turek said, creating a final product that looks something like a transparent, over-sized Tootsie Roll.
1/20/2012
Stem Cell Research Problems
Another one opinion on stem cell research:
http://shyfish.info/a-peek-at-each-side-in-the-stem-cell-studies-discussion/
A lot has been written and also said about stem cell research, and most of these trumpet about ultimately locating a cure for several ailments. Nevertheless, presently there are also camps that absolutely dismiss the stem cell research pros, while focusing on its purportedly damaging aspects. But before many of us live on the stem cell research disadvantages, allow us very first discuss exactly what come cellular material are regarding. Originate cells produce fresh blood vessels tissues, which is why they will are considered as the blood’s human being building blocks. The thing that makes come cells particular is the simple fact that they can possibly become various kinds of cells, which usually in switch may be developed into specialized cellular material that may be used by scientists in their own search for ways to repair bodily organs that have by now sustained harm.
Of the a couple of vast forms of stem tissues observed in mammals, embryonic base cells are the versions that could possibly grow into other kinds of tissues. Very easily stored in a laboratory, embryonic base cellular material, are additionally special in the feeling that they are able to identical them selves in countless amounts, along with forever with that.
This quality on your own means they are ideal for scientific utilize along with ongoing stem cell research. Most of this reports have already proven that serious diseases similar to ms, Parkinson's along with Alzheimer's is treatable with the assistance of come tissue. That perhaps retains the potential to the treatment of heart disease, strokes, kidney disease, liver disease, pancreas disease and also arthritis, as well as discovering a treat for cancer malignancy.
But for almost all the stem cell research pros, some groupings notice the concerns in the idea. Much of the stem cell research downsides that that they are professing are moral in character. These people say that because embryonic come cells are in truth little embryos, just what stem mobile researchers are tinkering with is living. Almost all of those who take this distinct argument are those which consider that existence starts with conception, certainly not at start, as just what is typically accepted.
The problem of cloning has also hounded stem cellular scientists, which profess they only rely on them for research functions, not necessarily to clone another individual. Nevertheless whichever the problems for or versus stem cell research are, the truth remains that teens is on the verge involving a main health-related as well as scientific jump, one that can modify the globe once and for all.
1/06/2012
Stemmy!111 ^_^
So cuuuuute!11111
And more about Stemmy:
Aaaaaannnnnddddd moooooooreeee:
And more about Stemmy:
Aaaaaannnnnddddd moooooooreeee:
Pictures from http://www.terry.ubc.ca/2009/08/07/faq-stem-cells-sa-mix/, moreover you can find text there.
12/27/2011
Stem Cells!
Beautiful Stem Cells! Thnx to
http://stemcelldaily.com/stem-cells-are-purdy-pics/ :

Neurosphere composed of neural precursor cells as captured by a fluorescent microscope. The cells, allowed to attach to a substrate, have begun to send out long processes that will eventually become the axons of the mature neurons.
The image was taken in the lab of Martin Pera at the University of Southern California.

A confocal microscopic image of a neurosphere, a ball of human embryonic stem cells giving rise to nerve cells. The nuclei of the neurons are shown in blue, while the axons are shown in red.
The image was taken in the lab of Juan Carlos Izpisua Belmonte at The Salk Institute for Biological Studies.

Color-enhanced electron microscopic image of mouse embryonic stem cells growing on a bed of silicon nanotubes.
The image was taken in the lab of Bruce Conklin at the Gladstone Institute for Cardiovascular Medicine.

Color-enhanced image taken by a scanning electron microscope of retinal pigment epithelial (RPE) cells derived from human embryonic stem cells. The cells are remarkably similar to normal RPE cells, having a hexagonal shape and growing in a single, well defined layer. These cells are the ones responsible for macular degeneration, the most common cause of blindness. CIRM scientists hope to one day treat macular degeneration with transplanted RPE cells derived from human embryonic stem cells.
The image was taken in the lab of David Hinton at the University of Southern California.
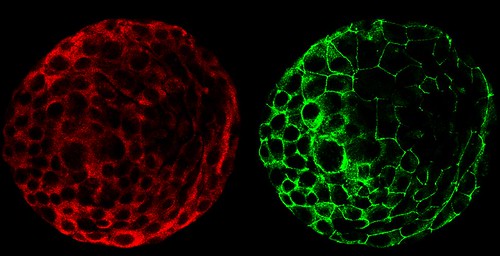

A composite of two images taken of a human embryo under different fluorescent wavelengths using a confocal microscope.
Fluorescent tags reveal that cells on the surface of the embryo are expressing human chorinoic gonadotropin (green tag) and an adhesion molecule (red tag)that helps them stick together.
The image was taken in the lab of Susan Fisher at the University of California, San Francisco.
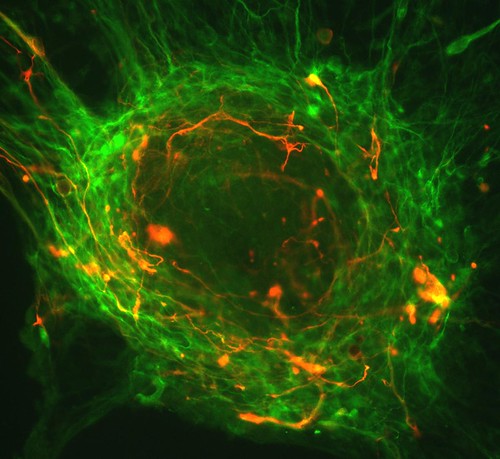

A fluorescent microscopic image of hundreds of human embryonic stem cells in various stages of differentiation into neurons. Some cells have become neurons (red), while others are still precursors of nerve cells (green). The yellow is an imaging artifact that results when cells in both stages are on top of each other.
The image was taken in the lab of Guoping Fan at the University of California, Los Angeles.

Colonies of human embryonic stem cells as seen with a fluorescent microscope. Nuclei have been stained blue, while regions that appear pink and green have been stained with antibodies, indicating the cells’ pluripotency — that unique ability of stem cells to differentiate into a variety of cell types.
The image was taken in the lab of Prue Talbot at the University of California, Riverside.


Three neurons (red) derived from human embryonic stem cells (hESCs) as seen by a confocal microscope. Visible are neural cell bodies, complete with axons and dendrites (red), used for cell-to-cell communications, as well as undifferentiated hESCs (green).
The image was taken in the lab of Anirvan Ghosh at the University of California, San Diego.


A fluorescent microscopic image of a functional neuron with an axon (red) growing above the cell’s nucleus and three dendrites (red) below. Undifferentiated neural precursor cells (blue) are visible as are glia cells (green) that have differentiated from the same group of mouse neural stem cells.
The image was taken in the lab of Paul Knoepfler at the University of California, Davis.


Two neurospheres, compact masses of neuron precursor cells, derived from human embryonic stem cells, as captured by a fluorescent microscope. Differentiated neurons, whose nuclei are shown in red, have begun to extend neuronal processes (green) toward one another, forming connections.

Neurosphere composed of neural precursor cells as captured by a fluorescent microscope. The cells, allowed to attach to a substrate, have begun to send out long processes that will eventually become the axons of the mature neurons.
The image was taken in the lab of Martin Pera at the University of Southern California.

A confocal microscopic image of a neurosphere, a ball of human embryonic stem cells giving rise to nerve cells. The nuclei of the neurons are shown in blue, while the axons are shown in red.
The image was taken in the lab of Juan Carlos Izpisua Belmonte at The Salk Institute for Biological Studies.

Color-enhanced electron microscopic image of mouse embryonic stem cells growing on a bed of silicon nanotubes.
The image was taken in the lab of Bruce Conklin at the Gladstone Institute for Cardiovascular Medicine.

Color-enhanced image taken by a scanning electron microscope of retinal pigment epithelial (RPE) cells derived from human embryonic stem cells. The cells are remarkably similar to normal RPE cells, having a hexagonal shape and growing in a single, well defined layer. These cells are the ones responsible for macular degeneration, the most common cause of blindness. CIRM scientists hope to one day treat macular degeneration with transplanted RPE cells derived from human embryonic stem cells.
The image was taken in the lab of David Hinton at the University of Southern California.


A composite of two images taken of a human embryo under different fluorescent wavelengths using a confocal microscope.
Fluorescent tags reveal that cells on the surface of the embryo are expressing human chorinoic gonadotropin (green tag) and an adhesion molecule (red tag)that helps them stick together.
The image was taken in the lab of Susan Fisher at the University of California, San Francisco.


A fluorescent microscopic image of hundreds of human embryonic stem cells in various stages of differentiation into neurons. Some cells have become neurons (red), while others are still precursors of nerve cells (green). The yellow is an imaging artifact that results when cells in both stages are on top of each other.
The image was taken in the lab of Guoping Fan at the University of California, Los Angeles.

Colonies of human embryonic stem cells as seen with a fluorescent microscope. Nuclei have been stained blue, while regions that appear pink and green have been stained with antibodies, indicating the cells’ pluripotency — that unique ability of stem cells to differentiate into a variety of cell types.
The image was taken in the lab of Prue Talbot at the University of California, Riverside.


Three neurons (red) derived from human embryonic stem cells (hESCs) as seen by a confocal microscope. Visible are neural cell bodies, complete with axons and dendrites (red), used for cell-to-cell communications, as well as undifferentiated hESCs (green).
The image was taken in the lab of Anirvan Ghosh at the University of California, San Diego.


A fluorescent microscopic image of a functional neuron with an axon (red) growing above the cell’s nucleus and three dendrites (red) below. Undifferentiated neural precursor cells (blue) are visible as are glia cells (green) that have differentiated from the same group of mouse neural stem cells.
The image was taken in the lab of Paul Knoepfler at the University of California, Davis.


Two neurospheres, compact masses of neuron precursor cells, derived from human embryonic stem cells, as captured by a fluorescent microscope. Differentiated neurons, whose nuclei are shown in red, have begun to extend neuronal processes (green) toward one another, forming connections.
The image was taken in the lab of Fred H. Gage at the Salk Institute for Biological Studies.

Neurospheres made up of neural stem cells derived from human embryonic stem cells captured using fluorescence microscopy. Some cells (green) are destined to become neurons; others have yet to differentiate (red) or are in transition (yellow).
This photo was taken in the lab of Brian Cummings at the University of California, Irvine.

A composite image of frames taken of a developing human embryo captured using time-lapse video microscopy and a phase contrast microscope. The earliest frame is of a three-day-old embryo and appears in the upper left corner. An image of a five-day-old embryo appears in the lower right.
This photo was taken in the lab of Susan Fisher at the University of California, San Francisco.
You can find out more information about the contest here.

Neurospheres made up of neural stem cells derived from human embryonic stem cells captured using fluorescence microscopy. Some cells (green) are destined to become neurons; others have yet to differentiate (red) or are in transition (yellow).
This photo was taken in the lab of Brian Cummings at the University of California, Irvine.

A composite image of frames taken of a developing human embryo captured using time-lapse video microscopy and a phase contrast microscope. The earliest frame is of a three-day-old embryo and appears in the upper left corner. An image of a five-day-old embryo appears in the lower right.
This photo was taken in the lab of Susan Fisher at the University of California, San Francisco.
You can find out more information about the contest here.
12/26/2011
Embryonic stem cell
Isn't it gorgeous? :)
B0006219 Human embryonic stem cell
Credit: Annie Cavanagh. Wellcome Images
images@wellcome.ac.uk
http://images.wellcome.ac.uk
Copyrighted work available under Creative Commons by-nc-nd 2.0 UK, see http://images.wellcome.ac.uk/indexplus/page/Prices.html
B0006219 Human embryonic stem cell
Credit: Annie Cavanagh. Wellcome Images
images@wellcome.ac.uk
http://images.wellcome.ac.uk
Copyrighted work available under Creative Commons by-nc-nd 2.0 UK, see http://images.wellcome.ac.uk/indexplus/page/Prices.html
12/25/2011
A new study published in Nature reports that scientists have been able to grow working pituitary glands from embryonic stem cells from mice
The pituitary gland is a pea-sized endocrine gland at the base of the brain and secretes different hormones including Human Growth Hormone, or HGH and thyroid-stimulating hormone, or TSH. The release of these hormones plays a role in things such as growth, blood pressure, pregnancy and thyroid function. Inadequate release of certain hormones by the pituitary gland can lead to various hormone disorders.
The team of researchers, led by Dr. Yoshiki Sasai from the RIKEN Centre for Developmental Biology in Kobe, Japan has created a way to grow these little organs using embryonic stem cells from mice. Using a three dimensional culture, the team arranged the mouse stem cells in a way that mimicked the way a pituitary gland grows naturally in the embryo.
When the pituitary gland grows in the embryo, it is made from two different tissue types in the brain. Where these two different tissues come together is where the pituitary gland forms. The researchers set up the culture so these two tissues would come together similar to the way they do in the brain. The fold of tissue known as Rathke’s pouch formed naturally and grew into the pituitary gland. This pituitary gland took three weeks to grow and included all the cell types that are found in a normal pituitary gland.
The researchers then transplanted the tissue into mice that had pituitary problems and the hormone levels soon returned to normal.
While these pituitary glands were created with embryonic stem cells, the researchers believe they can use the same process successfully with stem cells taken from adults and avoid possible ethical concerns with the use of embryonic stem cells.
Research is planned to develop human pituitary glands though Sasai says that will take another four to five years. If successful, these lab-grown pituitary glands could be used to replace ones that have been damaged or do not function properly.
More information: Self-formation of functional adenohypophysis in three-dimensional culture, Nature (2011) doi:10.1038/nature10637
© 2011 PhysOrg.com
Thnx to http://medicalxpress.com/news/2011-11-pituitary-glands-embryonic-stem-cells.html for the article
The team of researchers, led by Dr. Yoshiki Sasai from the RIKEN Centre for Developmental Biology in Kobe, Japan has created a way to grow these little organs using embryonic stem cells from mice. Using a three dimensional culture, the team arranged the mouse stem cells in a way that mimicked the way a pituitary gland grows naturally in the embryo.
When the pituitary gland grows in the embryo, it is made from two different tissue types in the brain. Where these two different tissues come together is where the pituitary gland forms. The researchers set up the culture so these two tissues would come together similar to the way they do in the brain. The fold of tissue known as Rathke’s pouch formed naturally and grew into the pituitary gland. This pituitary gland took three weeks to grow and included all the cell types that are found in a normal pituitary gland.
The researchers then transplanted the tissue into mice that had pituitary problems and the hormone levels soon returned to normal.
While these pituitary glands were created with embryonic stem cells, the researchers believe they can use the same process successfully with stem cells taken from adults and avoid possible ethical concerns with the use of embryonic stem cells.
Research is planned to develop human pituitary glands though Sasai says that will take another four to five years. If successful, these lab-grown pituitary glands could be used to replace ones that have been damaged or do not function properly.
More information: Self-formation of functional adenohypophysis in three-dimensional culture, Nature (2011) doi:10.1038/nature10637
Abstract
The adenohypophysis (anterior pituitary) is a major centre for systemic hormones. At present, no efficient stem-cell culture for its generation is available, partly because of insufficient knowledge about how the pituitary primordium (Rathke’s pouch) is induced in the embryonic head ectoderm. Here we report efficient self-formation of three-dimensional adenohypophysis tissues in an aggregate culture of mouse embryonic stem (ES) cells. ES cells were stimulated to differentiate into non-neural head ectoderm and hypothalamic neuroectoderm in adjacent layers within the aggregate, and treated with hedgehog signalling. Self-organization of Rathke’s-pouch-like three-dimensional structures occurred at the interface of these two epithelia, as seen in vivo, and various endocrine cells including corticotrophs and somatotrophs were subsequently produced. The corticotrophs efficiently secreted adrenocorticotropic hormone in response to corticotrophin releasing hormone and, when grafted in vivo, these cells rescued the systemic glucocorticoid level in hypopituitary mice. Thus, functional anterior pituitary tissue self-forms in ES cell culture, recapitulating local tissue interactions.
© 2011 PhysOrg.com
Thnx to http://medicalxpress.com/news/2011-11-pituitary-glands-embryonic-stem-cells.html for the article
Subscribe to:
Posts (Atom)